Rabbit anti-human 12-LOX IgG
Description
Polyclonal antibody directed against human 12-Lipoxygenase (Platelet-type/arachidonate, 12-LOX, ALOX12). Rabbits were immunized with purified recombinant full-length human P-12-LOX, expressed in the baculovirus expression system. IgG was purified from rabbit serum by Protein A affinity chromatography. This polyclonal antibody reacts with both recombinant and native human 12-Lipoxygenase.
Presentation
Screw capped vial containing 500 μg of purified antibody; liquid in Tris-Glycin, pH 7.5, 0.5 M NaCl, 1mg/ml BSA, 0.02 % NaN3
Concentration
The IgG concentration is 7.5 mg/ml.
Storage and Stability
Store the antibody at 2°-8°C. For long term storage Reconstituted antibody should be aliquoted and stored at –20°C or colder. It is recommended to avoid freeze-thaw cycles.
Specificity
The antibody is specific for 12-LOX/ALOX12. No cross-reactivity was observed against other members of the Lipoxygenase family (5-LOX, 15-LOX-1, 15-LOX-2, 12R-LOX, and eLOX) when tested in ELISA and native PAGE.
Applications
ELISA
The antibody can be used as detector or coating antibody in ELISAs. An antibody concentration of
1-10 µg/ml is recommended.
Western Blot
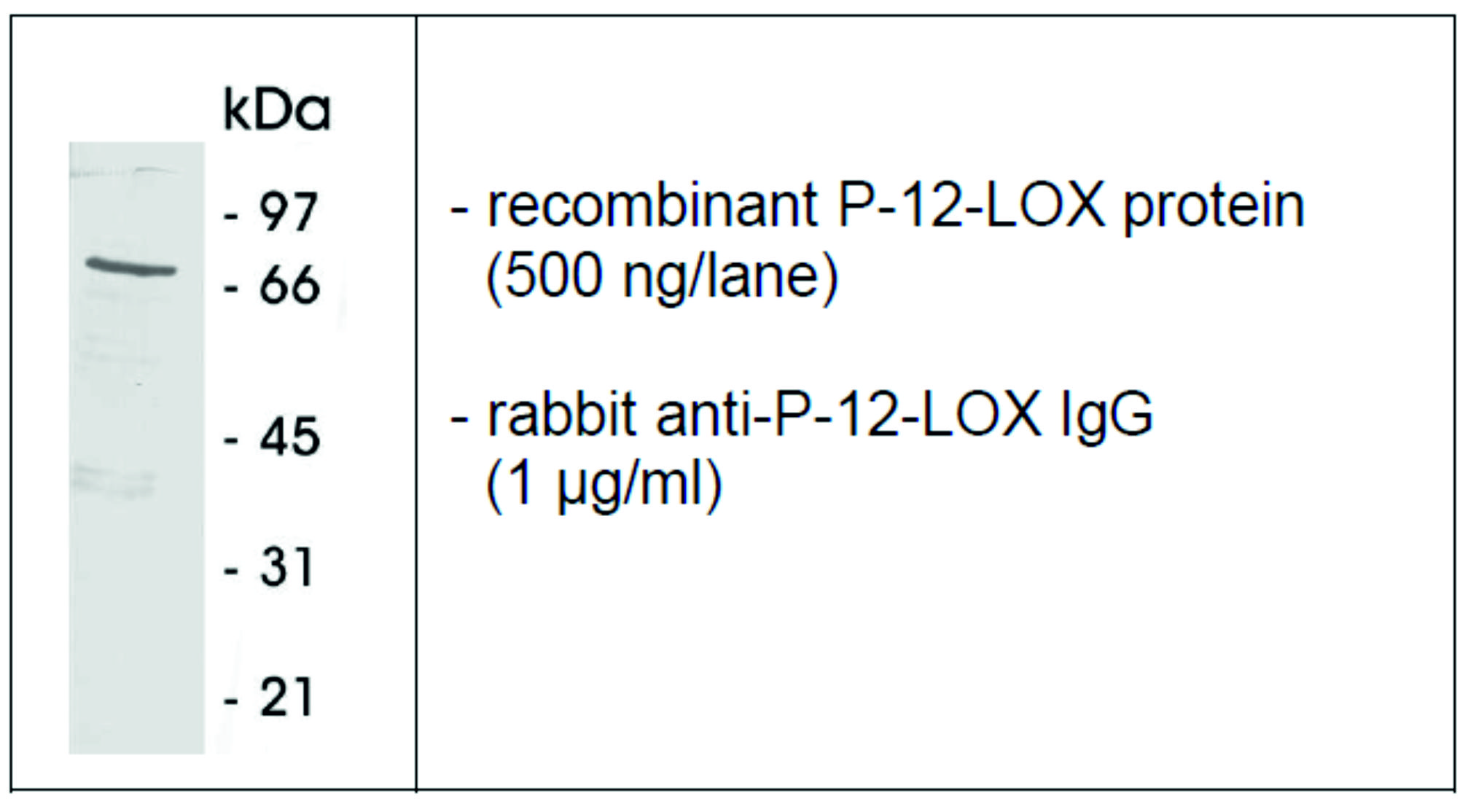
The antibody recognizes native and recombinant human P-12-LOX following SDS-PAGE under reducing conditions. A primary antibody concentration of 1-10 µg/ml is recommended.
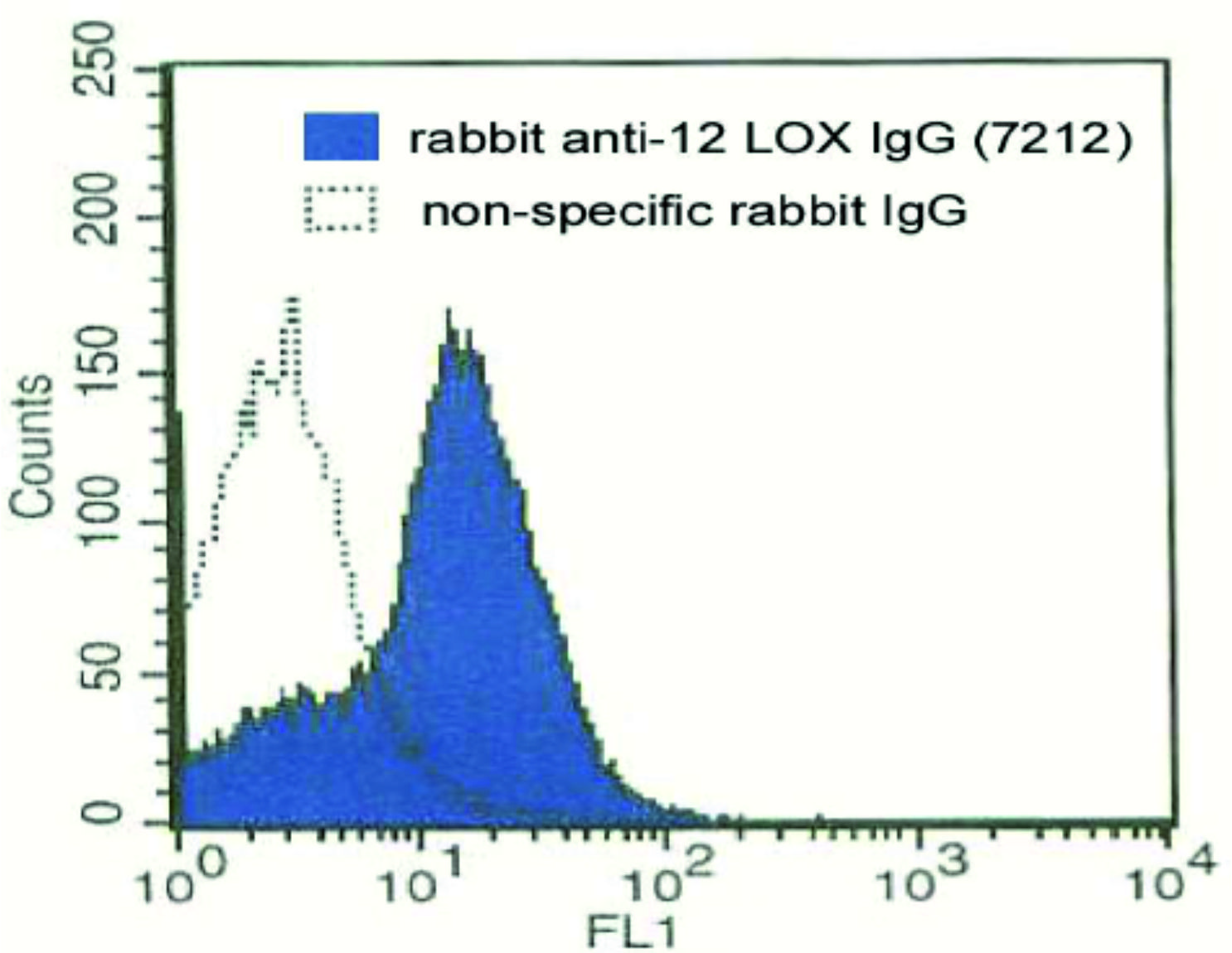
Flow cytometry
The antibody can be used for flow cytometry on formaldehyde-fixed and permeabilized human platelets. A primary antibody concentration of 1-10 µg/ml is recommended.
Immunohistochemistry
The antibody can be used for immune-histochemistry on formalin-fixed, paraffin-embedded tissue sections. A primary antibody concentration of 1-10 µg/ml is recommended.
Category: Research use only
Type: Antibody
Product Availability: Worldwide
Manufacturer: ImmBioMed GmbH & Co KG, Germany
For more information please click .pdf icon below.
Rabbit anti-human 12-LOX IgG
Cat.No. ADG7212
Article no.: 938274
Unit: 500 µg
Code: ADG7212
Manufacturer: ImmBioMed GmbH & Co. KG
References
- Human platelet 12-lipoxygenase, new findings about its activity, membrane binding and low-resolution structure. Aleem AM et al., E. J Mol Biol. 2008 Feb 8;376(1):193-209
- The naturally occurring Q261R variant of the human platelet 12-lipoxygenase exhibits a reduced catalytic activity but unaltered reaction specificity, substrate binding behavior and membrane association. Aleem AM et al., Int J Mol Med. 2009 Dec;24(6):759-764
- Platelet 12-lipoxygenase and stem cells in Barrett's esophagus. Jaśkiewicz K et al., Oncol Letters 2010 1: 789-791